April 2023
Rafal Nowak, MD, PhD Department of Ophthalmology, Military Institute of Medicine, Warsaw, Poland
Correspondence to: drrafal007@gmail.com Introduction Granulomatosis with polyangiitis (GPA; Wegener's granulomatosis) is a rare disease of unknown etiology. In its course, there is necrotizing inflammation of small and medium-sized blood vessels, as well as granuloma formation. Typically, it affects the upper and lower respiratory tracts, kidneys, and less commonly other locations. Standard treatment is based on immunosuppression, and if successful - leads to remissionI. GPA often causes lacrimal drainage obstruction at the level of the nasolacrimal ductII. This condition may cause excessive epiphora, as well as chronic and acute dacryocystitisIII. Nasolacrimal duct obstruction is routinely treated with the surgical procedure of dacryocystorhinostomy (DCR), which refers to the creation of a functional pathway for tears to drain into the nasal cavity by means of making an osteotomy and opening the lacrimal sac directly into the nasal cavity. It can be performed via an external or endonasal approach. DCR that has failed multiple times is uncommon and poses a surgical challengeIV. Unsuccessful dacryocystorhinostomy may result from chronic inflammatory changes in the nasal cavity, which are often found during GPAV. Another procedure used in the treatment of lacrimal drainage obstruction is endoscopic dacryoplasty (DCP). In this surgery a balloon catheter is introduced into the nasolacrimal duct and inflated to high pressure to dilate the stenosis. DCP with larger size balloon catheters was found by some authors to be successful also in management of failed DCR surgeriesVI. This study presents a case of a patient with diagnosed granulomatosis with polyangiitis, who underwent unsuccessful DCR surgeries in the past, and was finally operated on with the procedure of endoscopic dacryoplastyVII. Medical History A 73-year-old adult female suffering from granulomatosis with polyangiitis for 10 years, who was receiving immunosuppressive medication of rituximab (MabThera, Roche, Germany), presented with left-sided dacryocele with recurrent acute dacryocystitis episodes (Fig. 1). She had previously undergone a FESS surgery on both sides, and later a laser DCR procedure on the right side, followed by an external DCR on both sides - neither of them successful. However, the right side remained asymptomatic afterwards.
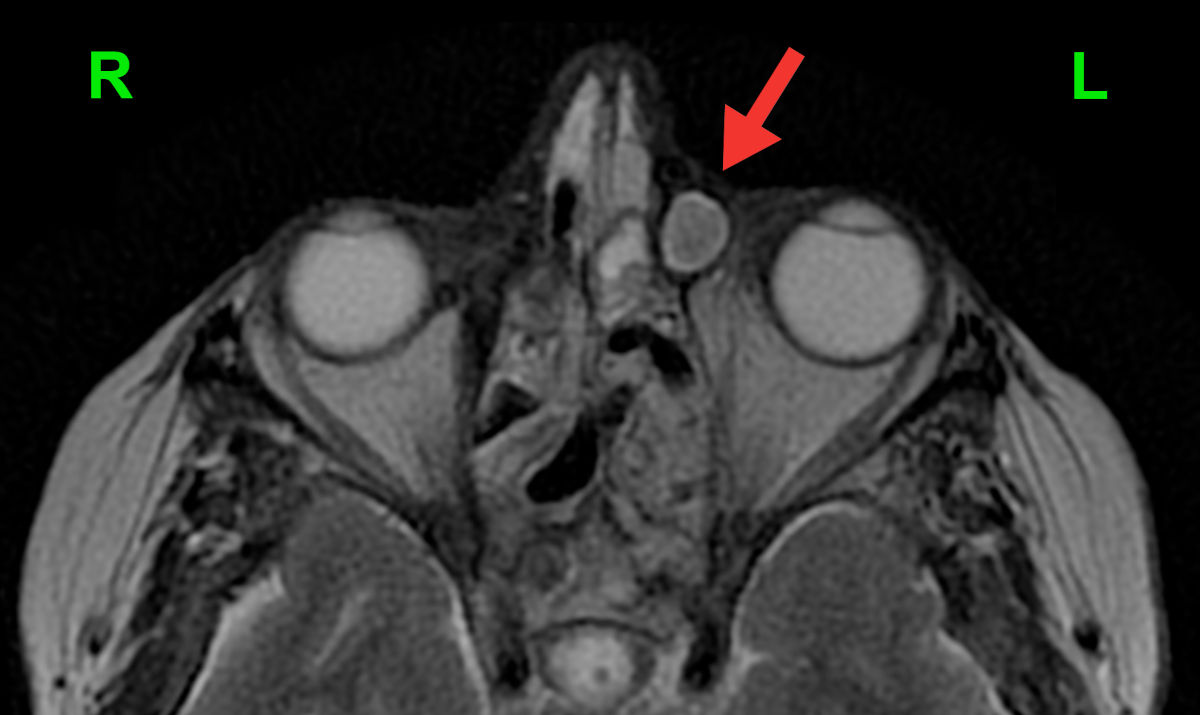 Figure 1. Axial MRI shows a well-defined left-sided dacryocele (enlarged lacrimal sac filled with discharge; red
arrow).
Examination
An endoscopy evaluation revealed extensive destruction with scarring in both nasal
cavities (Fig 2). On the left side, a small ostium was present. It was partially patent on irrigation under
pressure (Fig. 3).
Figure 1. Axial MRI shows a well-defined left-sided dacryocele (enlarged lacrimal sac filled with discharge; red
arrow).
Examination
An endoscopy evaluation revealed extensive destruction with scarring in both nasal
cavities (Fig 2). On the left side, a small ostium was present. It was partially patent on irrigation under
pressure (Fig. 3).
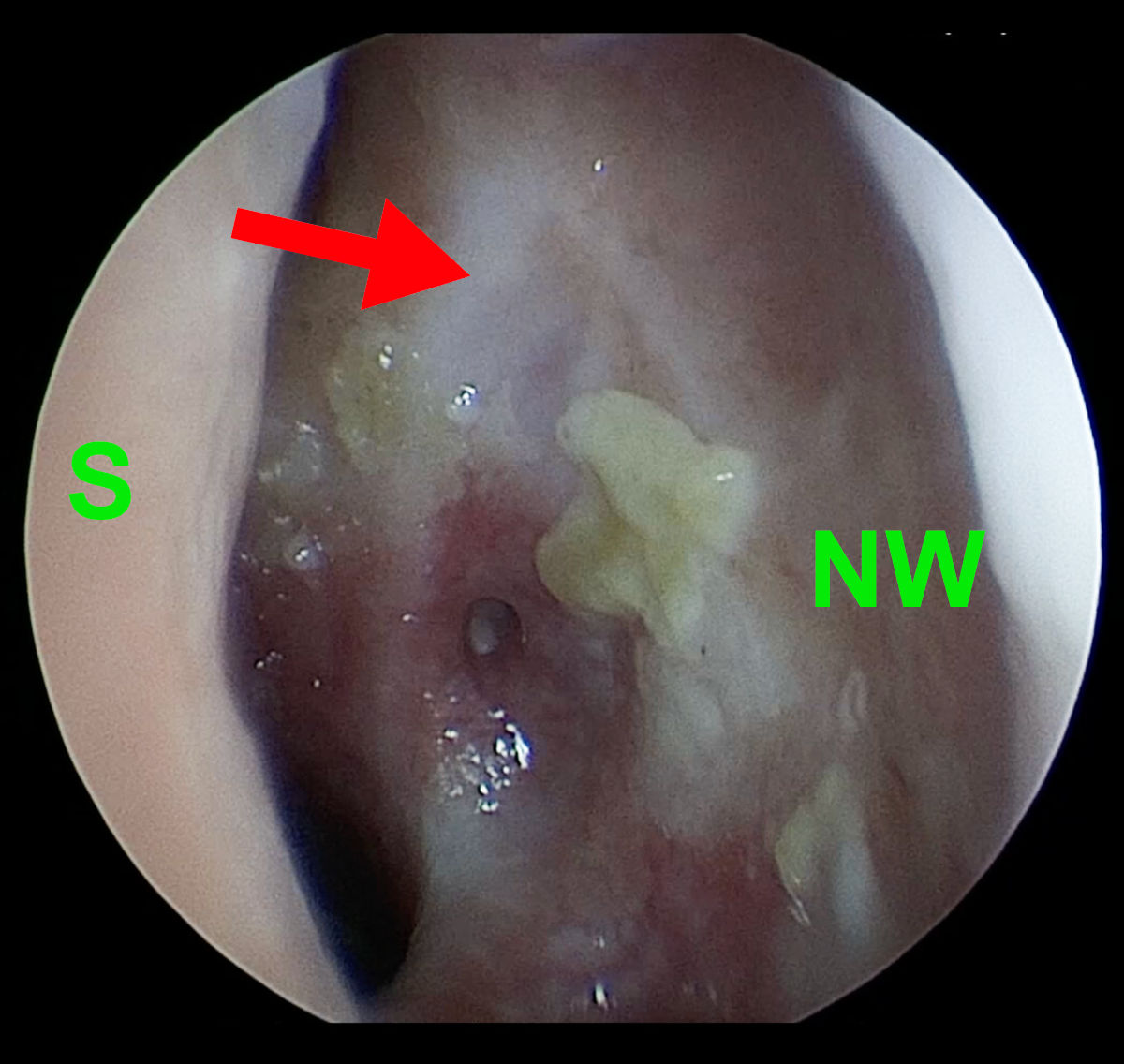 Figure 2. Left side endoscopy shows extensive destruction of anatomy (S- septum, NW- nasal wall, red arrow-
points at the scar after previous DCR surgery).
Figure 2. Left side endoscopy shows extensive destruction of anatomy (S- septum, NW- nasal wall, red arrow-
points at the scar after previous DCR surgery).
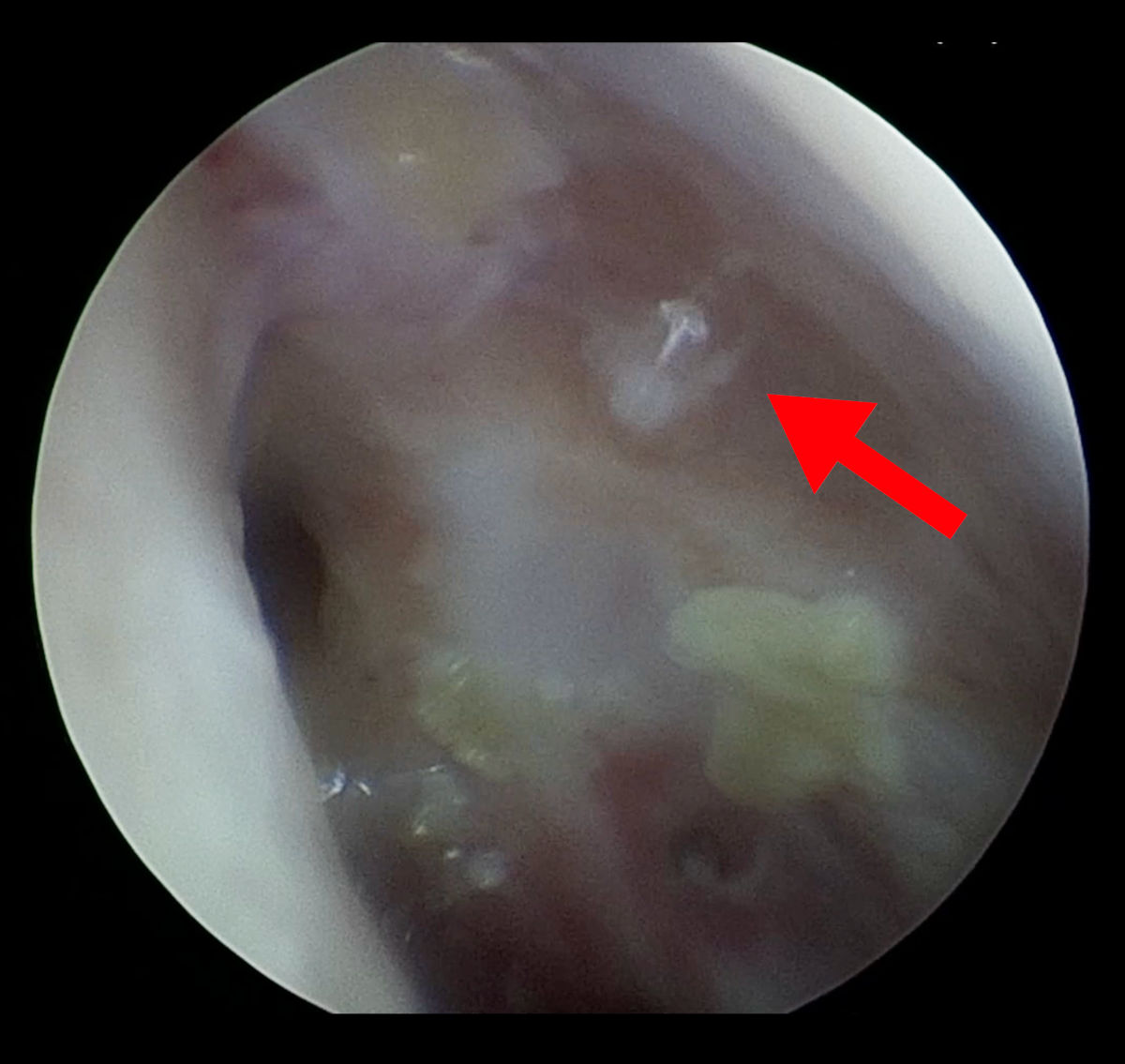 Figure 3. The same image as on Figure 2, note a thin trickle of fluid coming out from ostium (red arrow).
CT and MRI scans showed no aeration of the paranasal sinuses, foci of bone
destruction and a significant amount of scar tissue. The anatomy of the nasal cavity was completely disturbed
(Fig. 4-5)
Figure 3. The same image as on Figure 2, note a thin trickle of fluid coming out from ostium (red arrow).
CT and MRI scans showed no aeration of the paranasal sinuses, foci of bone
destruction and a significant amount of scar tissue. The anatomy of the nasal cavity was completely disturbed
(Fig. 4-5)
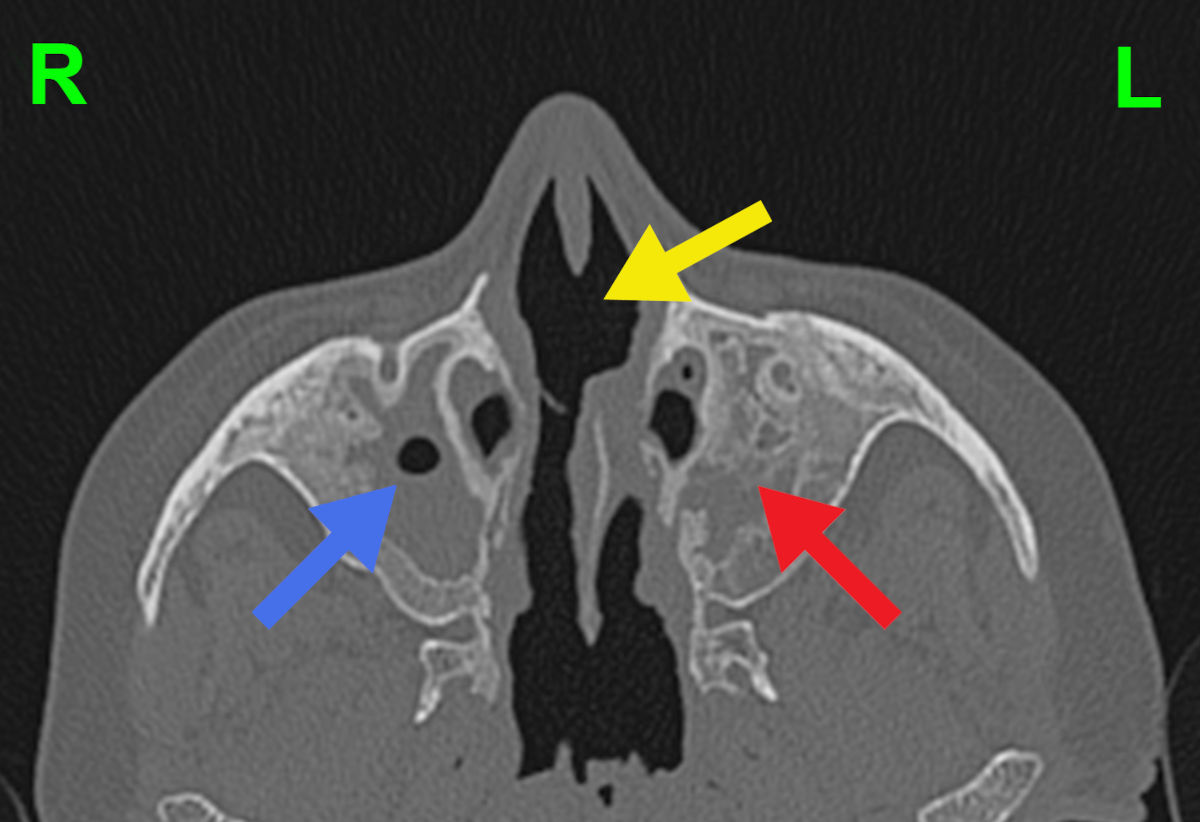 Figure 4. Axial CT scan shows residual aeration of the right maxillary sinus (blue arrow), bone destruction and
no aeration of the left maxillary sinus (red arrow), and destruction of the nasal septum (yellow arrow).
Figure 4. Axial CT scan shows residual aeration of the right maxillary sinus (blue arrow), bone destruction and
no aeration of the left maxillary sinus (red arrow), and destruction of the nasal septum (yellow arrow).
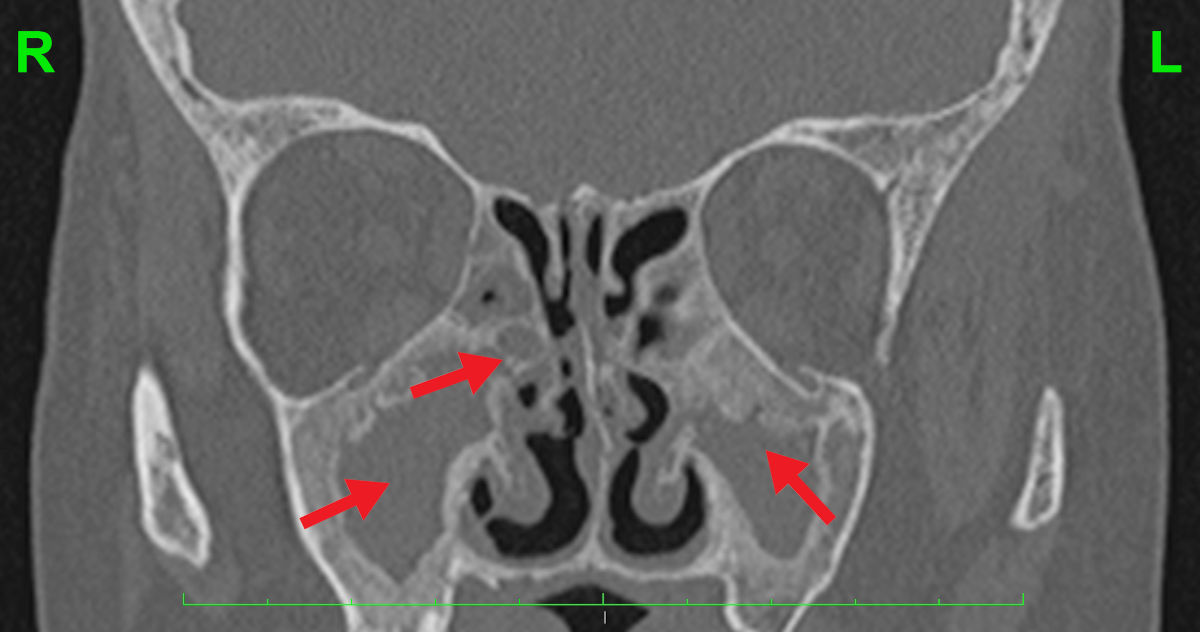 Figure 5. Coronal CT scan shows no aeration of the paranasal sinuses and deformation of the nasal cavity
anatomy.
These lesions were visualized with volumetric 3D-rendering of the CT and MRI
images with Medical Imaging
XR software (MedicalHolodeck, Switzerland), which allowed for better evaluation of
the pathologically altered craniofacial anatomy (Fig. 6-9).
Figure 5. Coronal CT scan shows no aeration of the paranasal sinuses and deformation of the nasal cavity
anatomy.
These lesions were visualized with volumetric 3D-rendering of the CT and MRI
images with Medical Imaging
XR software (MedicalHolodeck, Switzerland), which allowed for better evaluation of
the pathologically altered craniofacial anatomy (Fig. 6-9).
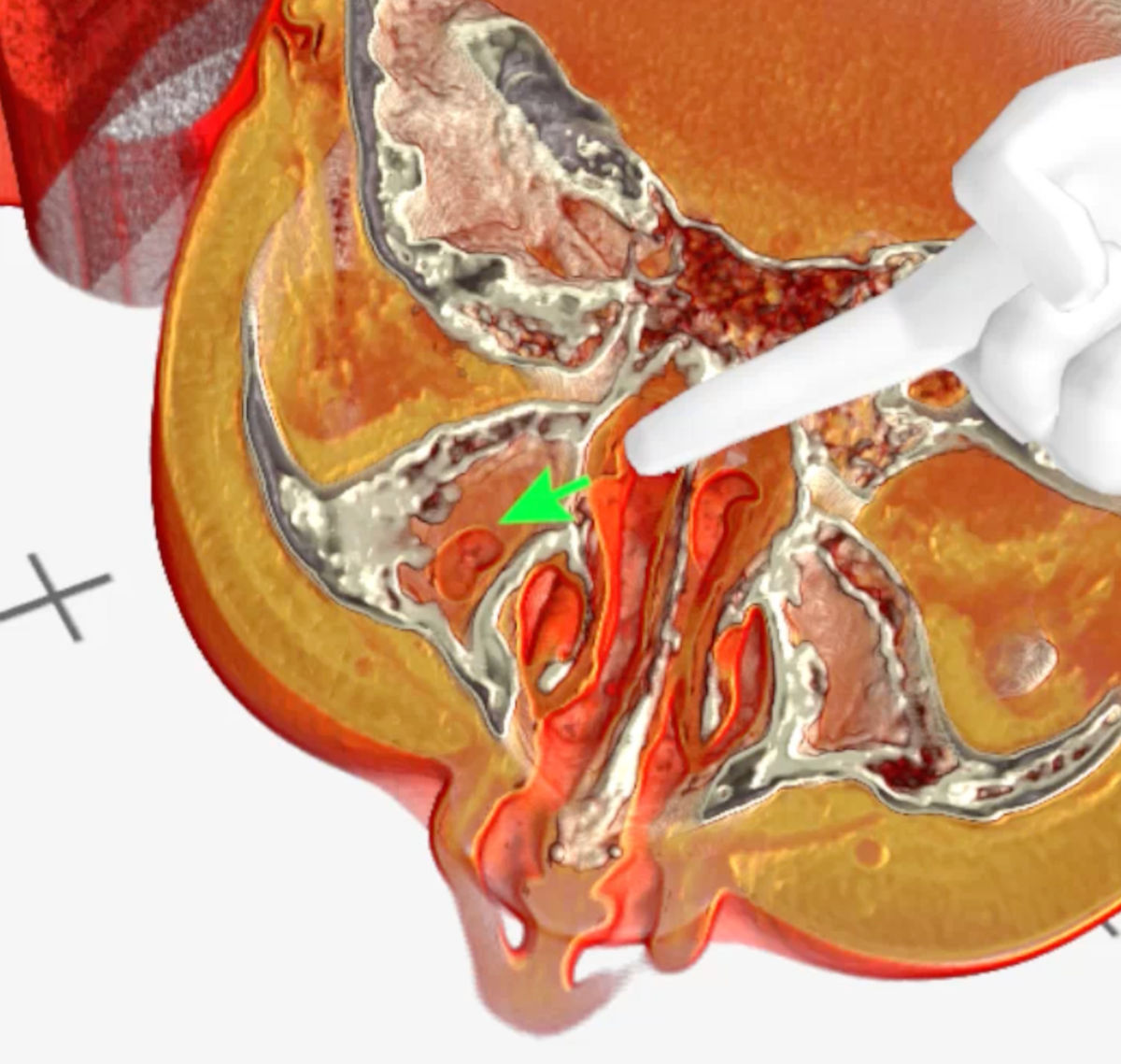 Figure 6. Medical Imaging XR 3D-rendered CT shows residual aeration of the right maxillary sinus (green arrow).
Figure 6. Medical Imaging XR 3D-rendered CT shows residual aeration of the right maxillary sinus (green arrow).
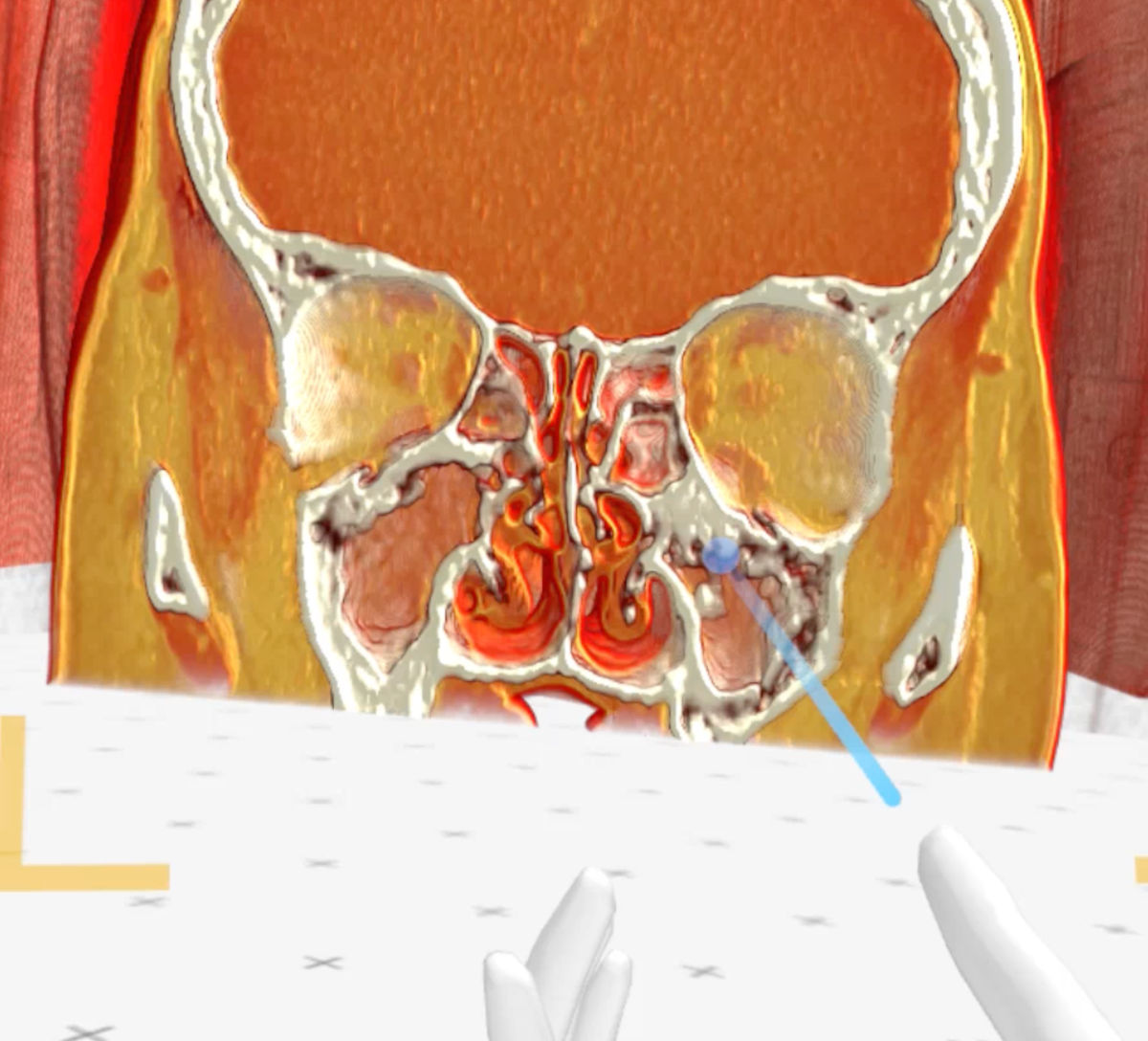 Figure 7. Medical Imaging XR 3D-rendered CT shows bone destruction of the left maxillary sinus walls.
Figure 7. Medical Imaging XR 3D-rendered CT shows bone destruction of the left maxillary sinus walls.
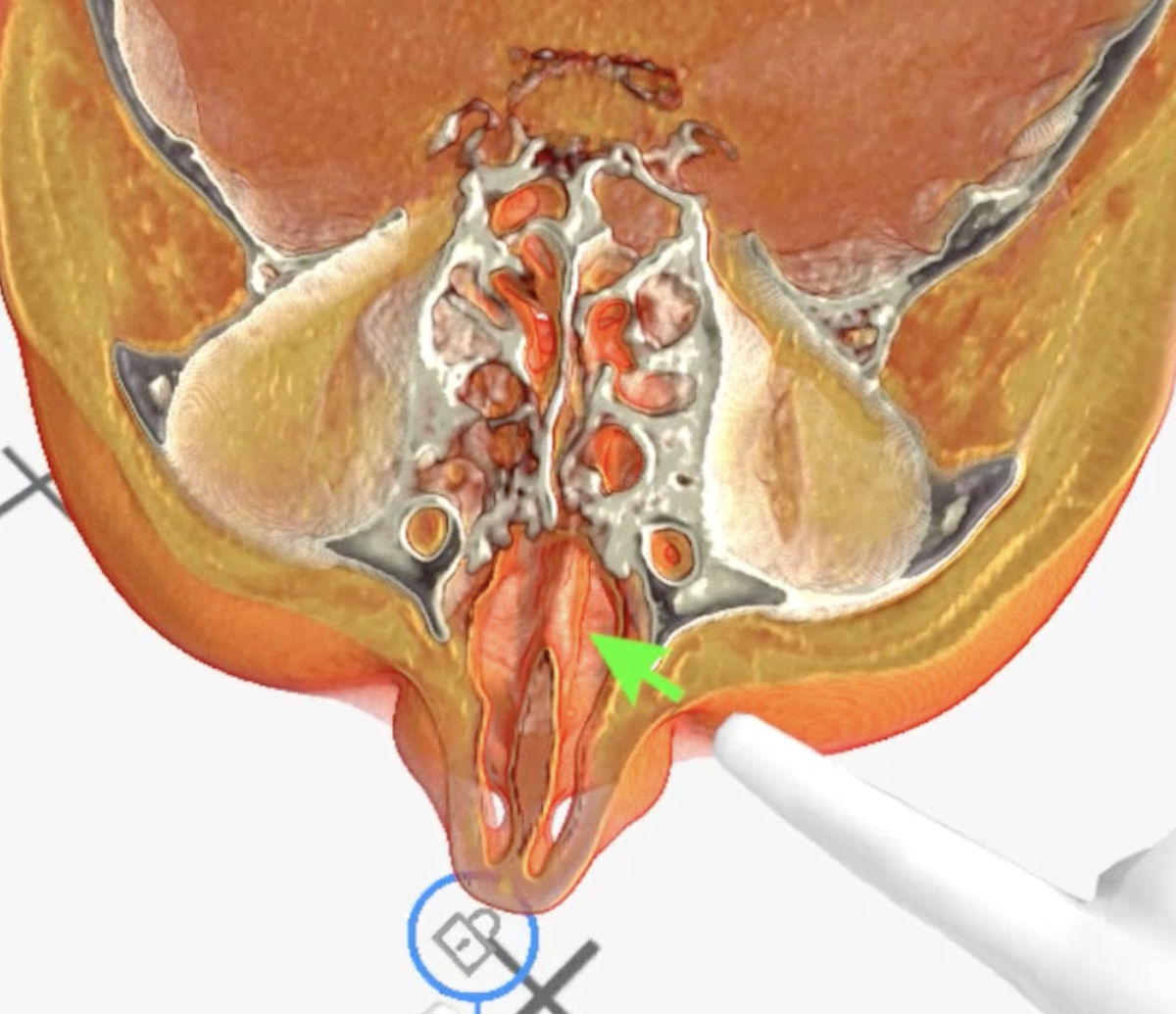 Figure 8. Medical Imaging XR 3D-rendered CT shows nasal septum defect (green arrow).
Figure 8. Medical Imaging XR 3D-rendered CT shows nasal septum defect (green arrow).
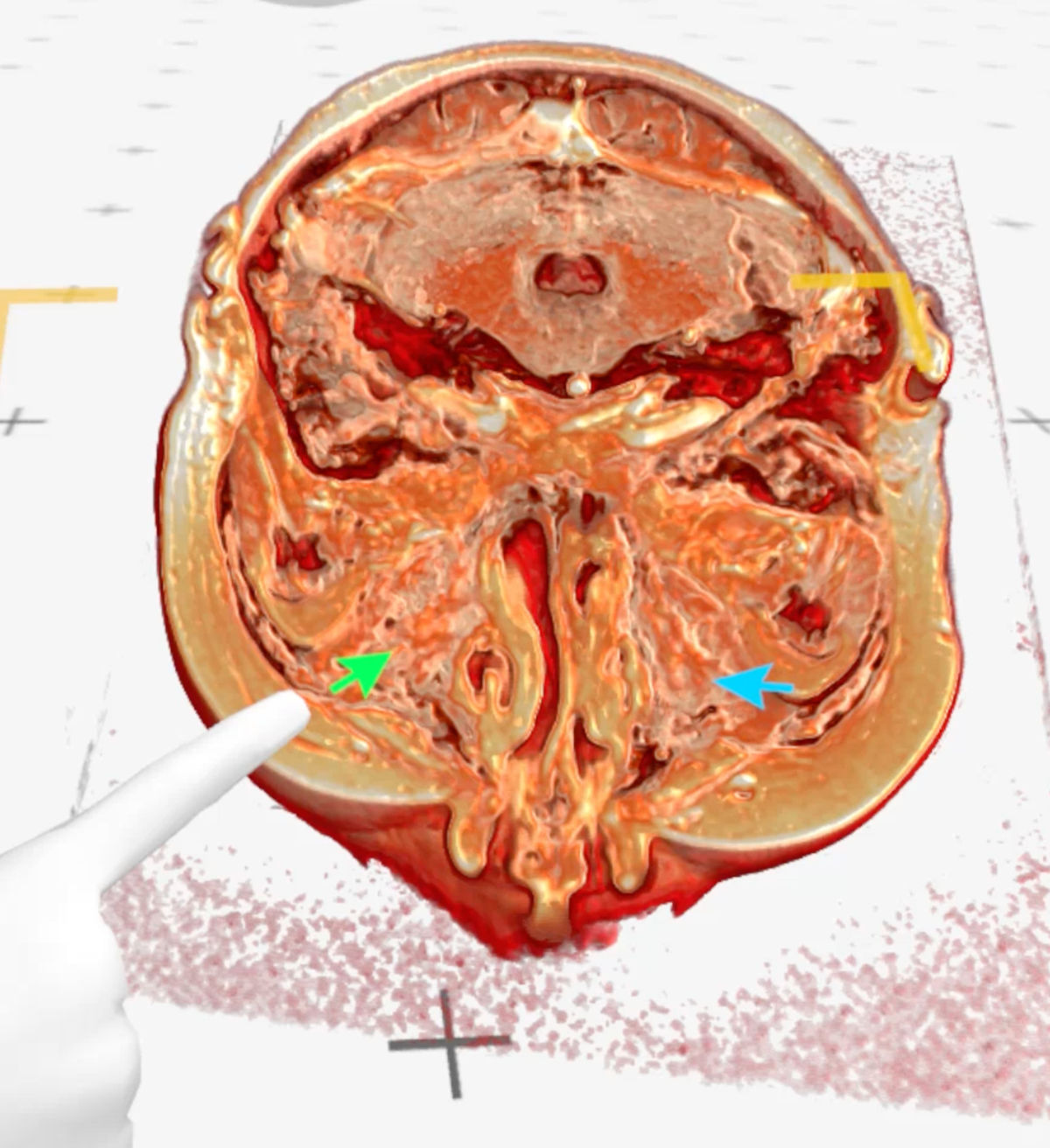 Figure 9. Medical Imaging XR 3D-rendered MRI shows maxillary sinuses completely filled with soft tissue (green
arrow - right maxillary sinus, blue arrow - left maxillary sinus).
Surgical Procedure
A 4 mm coronary balloon catheter (Boston Scientific, USA) was used to conduct a
balloon dacryoplasty on the patient's left side (Fig. 10). An incision was made in the scar tissue developed
after the previous DCR surgery (Fig. 11). The balloon catheter was introduced into the nasal cavity through the
lacrimal canaliculus, lacrimal sac and the incised scar tissue. The catheter was inflated to 8 atm for 90
seconds, then it was deflated and removed (Fig. 12-13). After debriding the neo-ostium's periphery scar tissues,
Mitomycin-C was applied. A significant internal common opening was visible in the immediate postoperative
period. The ostium was big, anatomically correct, and functionally patent at the 3-month follow-up. At six
months of follow-up, the patient was symptom-free and still receiving immunosuppressive treatment (Fig. 14-15).
Figure 9. Medical Imaging XR 3D-rendered MRI shows maxillary sinuses completely filled with soft tissue (green
arrow - right maxillary sinus, blue arrow - left maxillary sinus).
Surgical Procedure
A 4 mm coronary balloon catheter (Boston Scientific, USA) was used to conduct a
balloon dacryoplasty on the patient's left side (Fig. 10). An incision was made in the scar tissue developed
after the previous DCR surgery (Fig. 11). The balloon catheter was introduced into the nasal cavity through the
lacrimal canaliculus, lacrimal sac and the incised scar tissue. The catheter was inflated to 8 atm for 90
seconds, then it was deflated and removed (Fig. 12-13). After debriding the neo-ostium's periphery scar tissues,
Mitomycin-C was applied. A significant internal common opening was visible in the immediate postoperative
period. The ostium was big, anatomically correct, and functionally patent at the 3-month follow-up. At six
months of follow-up, the patient was symptom-free and still receiving immunosuppressive treatment (Fig. 14-15).
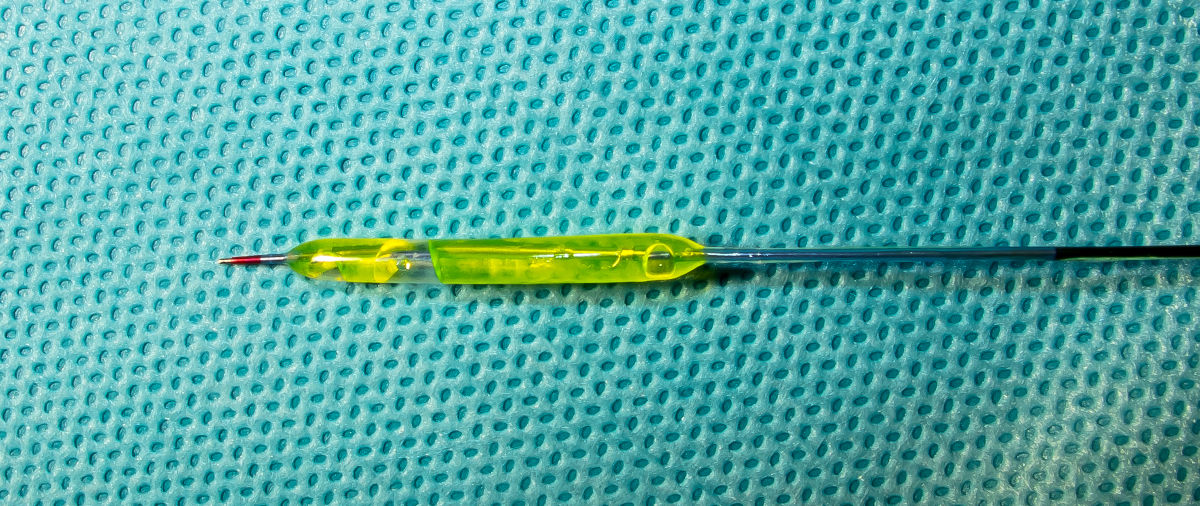 Figure 10. Coronary balloon catheter.
Figure 10. Coronary balloon catheter.
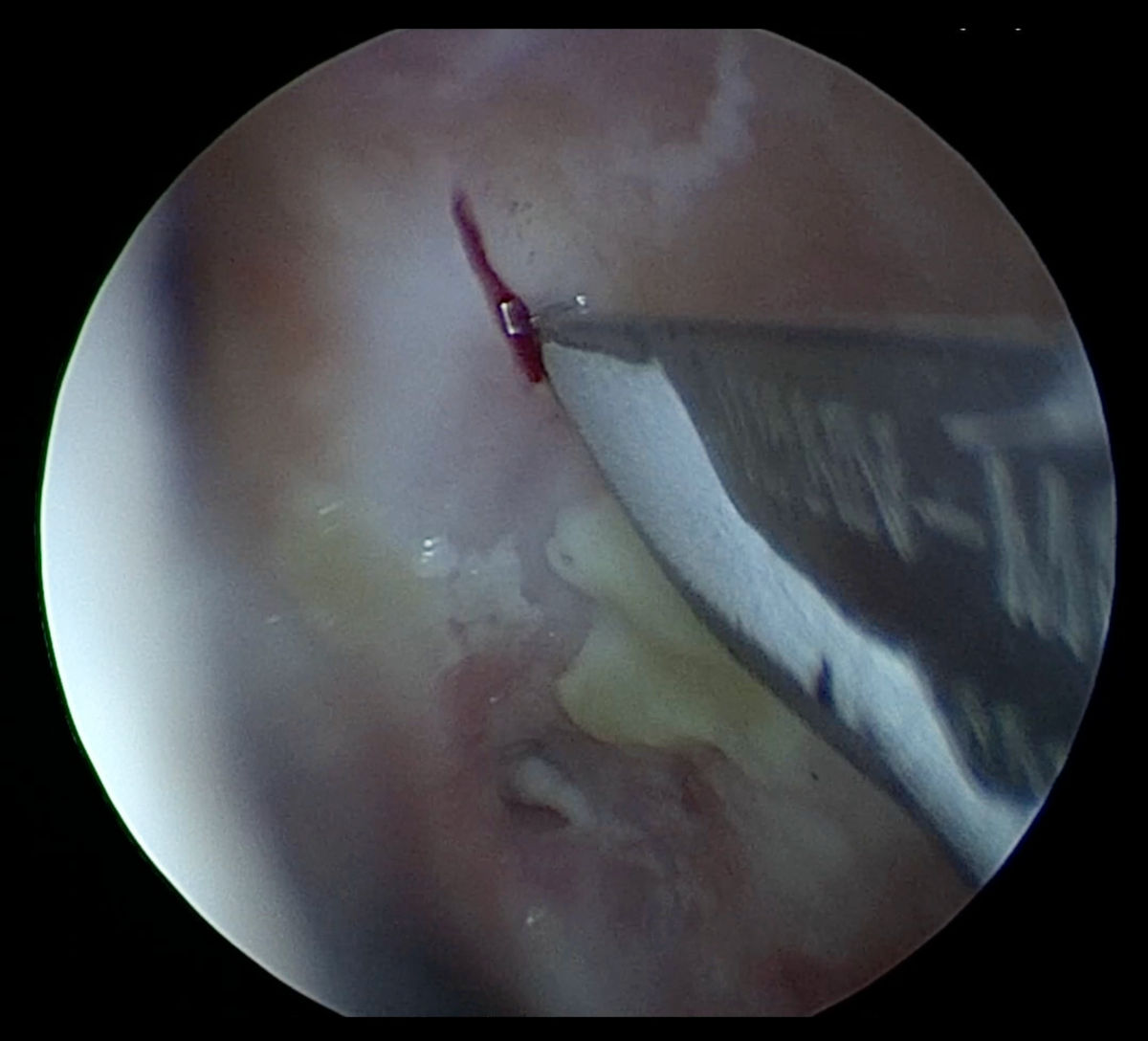 Figure 11. Endoscopy view of the left nasal cavity: incision being made in the scar tissue developed after
previous DCR surgery.
Figure 11. Endoscopy view of the left nasal cavity: incision being made in the scar tissue developed after
previous DCR surgery.
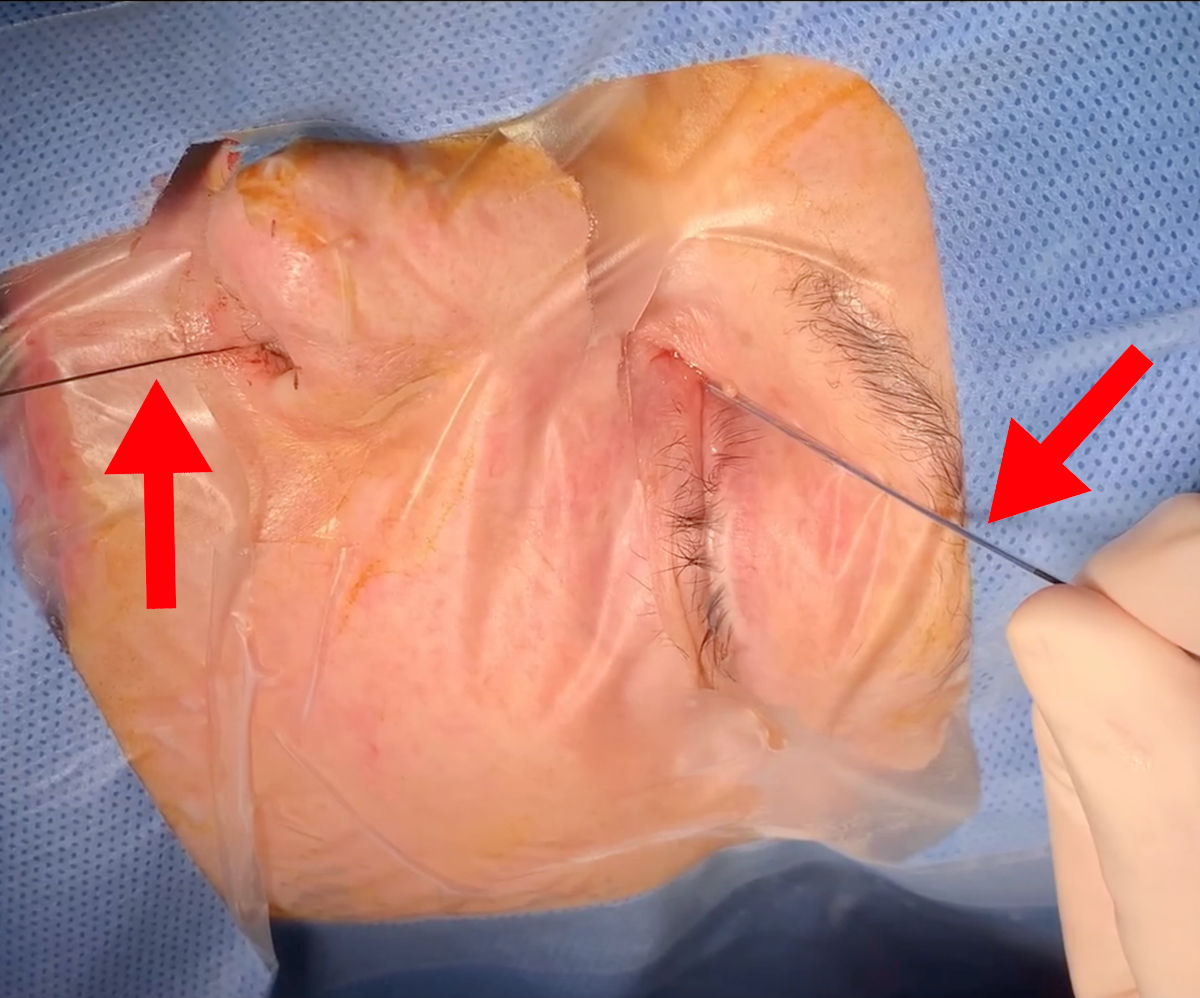 Figure 12. Balloon dacryoplasty: balloon catheter being introduced on a guide wire.
Figure 12. Balloon dacryoplasty: balloon catheter being introduced on a guide wire.
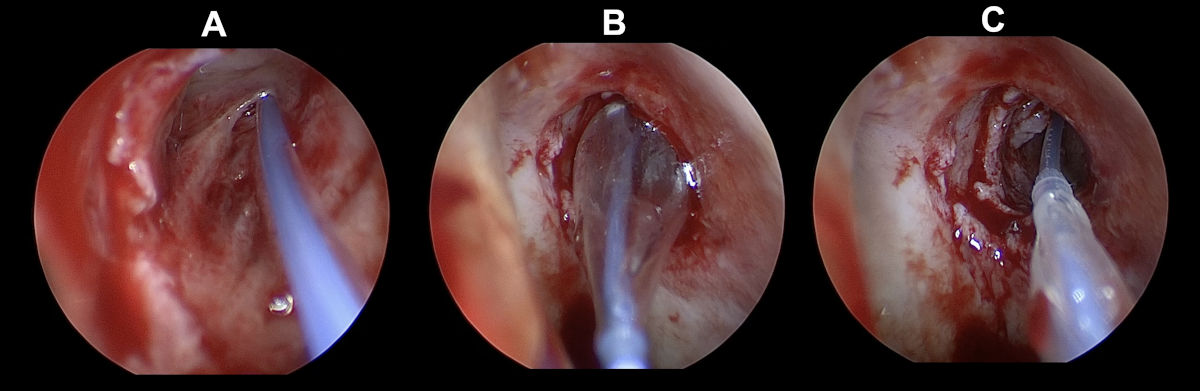 Figure 13. Endoscopy view of the left nasal cavity: balloon dacryoplasty (A- guide wire being introduced, B-
balloon catheter being inflated, C- balloon catheter being deflated; note enlarged ostium).
Figure 13. Endoscopy view of the left nasal cavity: balloon dacryoplasty (A- guide wire being introduced, B-
balloon catheter being inflated, C- balloon catheter being deflated; note enlarged ostium).
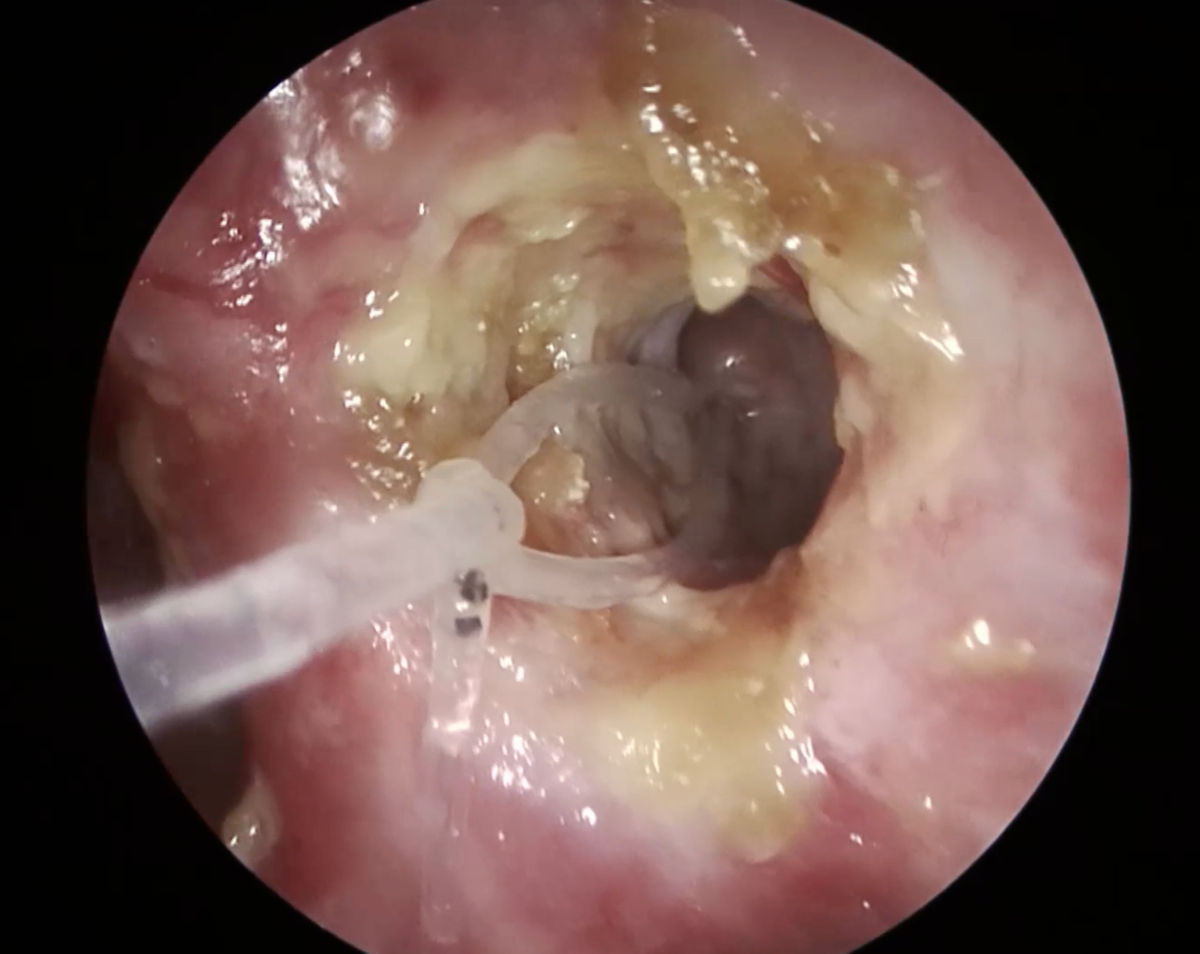 Figure 14. Endoscopy view of the left nasal cavity: ostium at 1 month after surgery (note the silicone stent
inside the ostium).
Figure 14. Endoscopy view of the left nasal cavity: ostium at 1 month after surgery (note the silicone stent
inside the ostium).
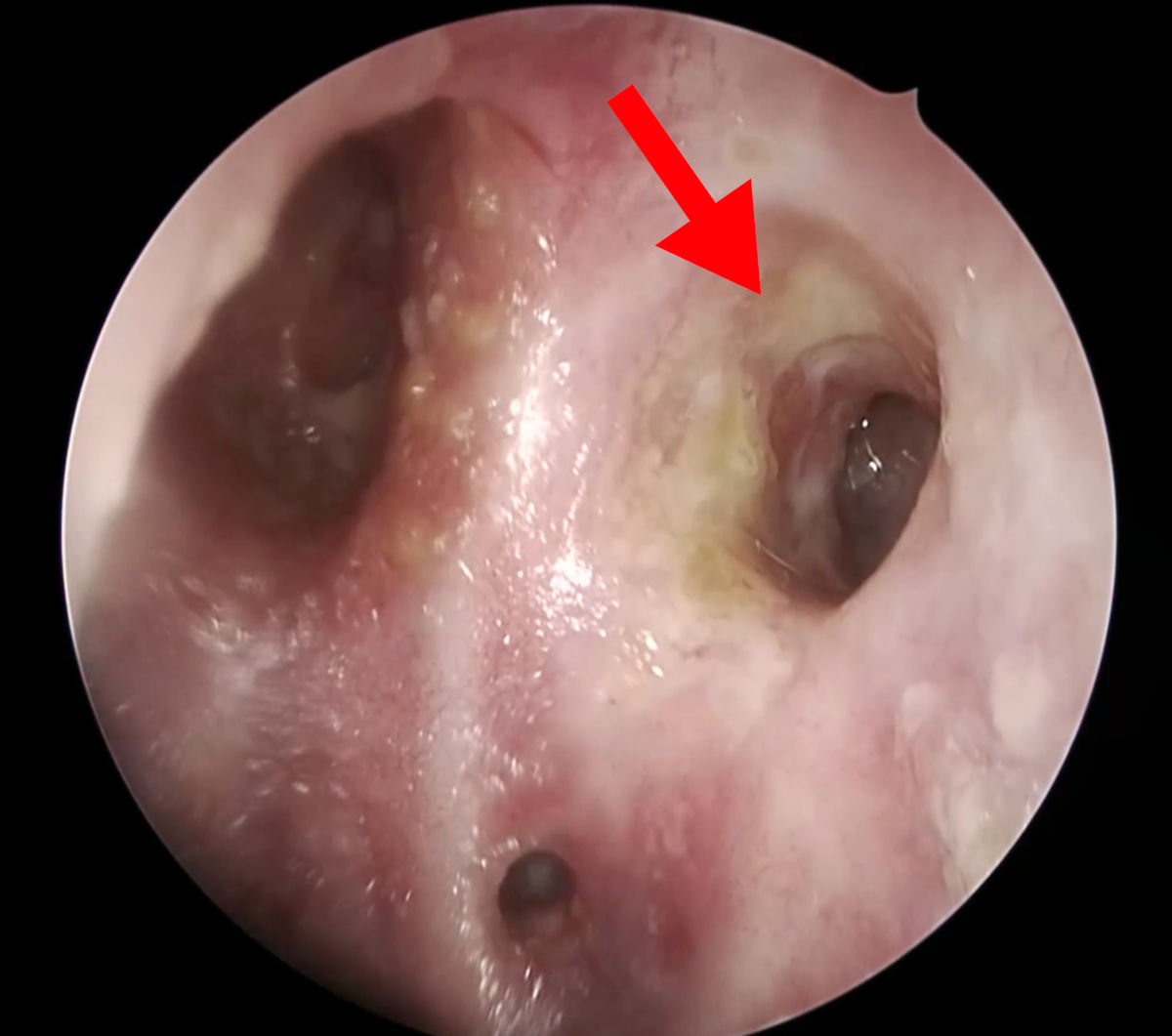 Figure 15. Endoscopy view of the left nasal cavity at 6 months after surgery (red arrow points at the patent
ostium).
Conclusions
Treatment of lacrimal drainage obstruction in cases of granulomatosis with
polyangiitis is challenging. It is possible to conduct it only during remission of the disease, when the process
of inflammation in the nasal cavity is not present, and the patient is under immunosuppressive treatment. The
current example shows that balloon-assisted dacryoplasty may be an effective solution for cases of failed DCR in
patients with Wegener's granulomatosis.
References
I. Garlapati P, Qurie A. Granulomatosis with Polyangiitis. [Updated 2022 Dec 5].
In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan.
Figure 15. Endoscopy view of the left nasal cavity at 6 months after surgery (red arrow points at the patent
ostium).
Conclusions
Treatment of lacrimal drainage obstruction in cases of granulomatosis with
polyangiitis is challenging. It is possible to conduct it only during remission of the disease, when the process
of inflammation in the nasal cavity is not present, and the patient is under immunosuppressive treatment. The
current example shows that balloon-assisted dacryoplasty may be an effective solution for cases of failed DCR in
patients with Wegener's granulomatosis.
References
I. Garlapati P, Qurie A. Granulomatosis with Polyangiitis. [Updated 2022 Dec 5].
In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan.
II. Sfiniadaki E, Tsiara I, Theodossiadis P, Chatziralli I. Ocular Manifestations of Granulomatosis with Polyangiitis: A Review of the Literature. Ophthalmol Ther. 2019 Jun;8(2):227-234. doi: 10.1007/s40123-019-0176-8. Epub 2019 Mar 15. PMID: 30875067; PMCID: PMC6513923.
III. Ali MJ. Principles and practice of lacrimal surgery. Singapore: Springer 2018
IV. Gupta N. Endoscopic dacryocystorhinostomy. Singapore: Springer: 2021
V. Kwan AS, Rose GE. Lacrimal drainage surgery in Wegener's granulomatosis. Br J Ophthalmol. 2000 Mar;84(3):329-31. doi: 10.1136/bjo.84.3.329. PMID: 10684848; PMCID: PMC1723393.
VI. Vinciguerra A, Indelicato P, Giordano Resti A, Bussi M, Trimarchi M. Long-term results of a balloon-assisted endoscopic approach in failed dacryocystorhinostomies. Eur Arch Otorhinolaryngol. 2022 Apr;279(4):1929-1935. doi: 10.1007/s00405-021-06975-3. Epub 2021 Jul 12. PMID: 34251520; PMCID: PMC8273032.
VII. Nowak R, Ali MJ. Endoscopic Coronary Catheter Dacryoplasty for Failed DCR in Wegener's Granulomatosis. Ocul Immunol Inflamm. 2023 Apr;31(3):599-600. doi: 10.1080/09273948.2022.2032200. Epub 2022 Feb 3. PMID: 35113738. For more information, contact info@medicalholodeck.com